|
Chapter
11 - Intermolecular Forces
11.1 A Molecular Comparison of Gases, Liquids, and Solids
• Physical properties of substances are understood in terms of
kinetic-molecular theory:
• Gases are highly compressible and assume the shape and volume
of their container.
• Gas molecules are far apart and do not interact much with one
another.
• Liquids are almost incompressible, assume the shape but not
the volume of the container.
• Liquids molecules are held together more closely than gas molecules
but not so rigidly that the molecules cannot slide past each other.
• Solids are incompressible and have a definite shape and volume.
• Solid molecules are packed closely together.
• The molecules are so rigidly packed that they cannot easily
slide past each other.
• Solids and liquids are condensed phases.
• Solids with highly ordered structures are said to be crystalline.
• Converting a gas into a liquid or solid requires the molecules
to get closer to each other.
• We can accomplish this by cooling or compressing the gas.
• Converting a solid into a liquid or gas requires the molecules
to move further apart.
• We can accomplish this by heating or reducing the pressure on
the gas.
• The forces holding solids and liquids together are called intermolecular
forces.
• Physical properties of liquids and solids are due to intermolecular
forces.
• These are forces between molecules.
11.2 Intermolecular Forces
• The attraction between molecules is an intermolecular force.
• Intermolecular forces are much weaker than ionic or covalent
bonds.
• When a substance melts or boils, intermolecular forces are broken.
• When a substances condenses, intermolecular forces are formed.
• Boiling points reflect intermolecular force strength.
• A high boiling point indicates strong attractive forces.
• Melting points also reflect the strength of attractive forces.
• A high melting point indicates strong attractive forces.
• van der Waals forces are the intermolecular forces that exist
between neutral molecules.
• These include London dispersion forces, dipole–dipole
forces, and hydrogen-bonding forces.
• Ion–dipole interactions are important in solutions.
• These are all weak (<15% as strong as a covalent or ionic
bond) electrostatic interactions.
Ion-Dipole Forces
• An ion–dipole force is an interaction between an ion (e.g.,
Na+) and the partial charge on the end of a polar molecule/dipole (e.g.,
water).
• It is especially important for solutions of ionic substances
in polar liquids.
• Example: NaCl(aq)
Dipole–Dipole Forces
• Dipole–dipole forces exist between neutral polar molecules.
• Polar molecules attract each other.
• The partially positive end of one molecule attracts the partially
negative end of another.
• Polar molecules need to be close together to form strong dipole–dipole
interactions.
• Dipole–dipole forces are weaker than ion–dipole
forces.
• If two molecules have about the same mass and size, then dipole–dipole
forces increase with increasing polarity.
• For molecules of similar polarity, those with smaller volumes
often have greater dipole–dipole attractions.
London-Dispersion Forces
• These are the weakest of all intermolecular forces.
• It is possible for two adjacent neutral molecules to affect
each other.
• The nucleus of one molecule (or atom) attracts the electrons
of the adjacent molecule (or atom).
• For an instant, the electron clouds become distorted.
• In that instant a dipole is formed (called an instantaneous
dipole).
• One instantaneous dipole can induce another instantaneous dipole
in an adjacent molecule (or atom).
• These two temporary dipoles attract each other.
• The attraction is called the London dispersion force, or simply
a dispersion force.
• London dispersion forces exist between all molecules.
• What affects the strength of a dispersion force?
• Molecules must be very close together for these attractive forces
to occur.
• Polarizability is the ease with which an electron distribution
can be deformed.
• The larger the molecule (the greater the number of electrons)
the more polarizable it is.
• London dispersion forces increase as molecular weight increases.
• London dispersion forces depend on the shape of the molecule.
• The greater the surface area available for contact, the greater
the dispersion forces.
• London dispersion forces between spherical molecules are smaller
than those between more cylindrically shaped molecules.
• Example: n-pentane vs. neopentane.
Hydrogen Bonding
• Experiments show that the boiling points of compounds with H–F,
H–O, and H–N bonds are abnormally high.
• Their intermolecular forces are abnormally strong.
• Hydrogen bonding is a special type of intermolecular attraction.
• This is a special case of dipole–dipole interactions.
• H-bonding requires:
• H bonded to a small electronegative element (most important
for compounds of F, O, and N).
• an unshared electron pair on a nearby small electronegative
ion or atom (usually F, O, or N on another molecule).
• Electrons in the H-X bond (X is the more electronegative element)
lie much closer to X than H.
• H has only one electron, so in the H-X bond, the H+ presents
an almost bare proton to the X-.
• Bond energies of hydrogen bonds vary from about 4 kJ/mol to
25 kJ/mol.
• They are much weaker than ordinary chemical bonds.
• Intermolecular and intramolecular hydrogen bonds have exceedingly
important biological significance.
• They are important in stabilizing protein structure, in DNA
structure and function, etc.
• An interesting consequence of H-bonding is that ice floats.
• The molecules in solids are usually more closely packed than
those in liquids.
• Therefore, solids are usually more dense than liquids.
• Ice is ordered with an open structure to optimize H-bonding.
• Water molecules in ice are arranged in an open, regular hexagon.
• Each d+ H points towards a lone pair on O.
• Therefore, ice is less dense than water.
• Ice floats, so it forms an insulating layer on top of lakes,
rivers, etc. Therefore, aquatic life can survive in winter.
• Water expands when it freezes.
• Frozen water in pipes may cause them to break in cold weather.
Comparing Intermolecular Forces
• Dispersion forces are found in all substances.
• Their strength depends on molecular shapes and molecular weights.
• Dipole–dipole forces add to the effect of dispersion forces.
• They are found only in polar molecules.
• H-bonding is a special case of dipole–dipole interactions.
• It is the strongest of the intermolecular forces involving neutral
species.
• H-bonding is most important for H compounds of N, O, and F.
• If ions are involved, ion–dipole (if a dipole is present)
and ionic bonding are possible.
• Ion–dipole interactions are stronger than H-bonds.
• Keep in mind that ordinary ionic or covalent bonds are much
stronger than these interactions!
FORWARD REFERENCES
• Molecular materials held together by intermolecular forces will
be called “soft” materials in Ch. 12.
• Intermolecular forces between polymer chains and in liquid crystals
will be mentioned in Ch. 12 (sections 12.6 and 12.8).
• Breaking of solute–solute and solvent–solvent intermolecular
forces and replacing them with solute–solvent interactions will
take place in the solution process (Ch. 13).
• The binding between the substrate and the active site in the
enzyme action thanks to the intermolecular forces will be discussed
in Ch. 14 (section 14.7).
• Hydrogen bonding and the formation of hydrated hydronium ions
will be discussed in Ch. 16 (section 16.2).
• Hydrogen bonding will be partially responsible for the relative
weakness of HF compared to the strength of other binary acids involving
halides (section 16.10).
• Hydrogen bonding and high heat capacity, high melting, and boiling
points of water will be mentioned again in Ch. 18 (section 18.5).
• Intermolecular attractions in ice will be discussed in Ch. 19
(section 19.3).
• Hydrogen bonding in alcohols will be discussed in Ch. 25 (section
25.5).
• Hydrogen bonding in the a helix of a protein will be discussed
in Ch. 25 (section 25.9).
11.3 Some Properties of Liquids
Viscosity
• Viscosity is the resistance of a liquid to flow.
• A liquid flows by sliding molecules over one another.
• Viscosity depends on:
• the attractive forces between molecules.
• The stronger the intermolecular forces, the higher the viscosity.
• the tendency of molecules to become entangled.
• Viscosity increases as molecules become entangled with one another.
• the temperature.
• Viscosity usually decreases with an increase in temperature.
Surface Tension
• Bulk molecules (those in the liquid) are equally attracted to
their neighbors.
• Surface molecules are only attracted inward towards the bulk
molecules.
• Therefore, surface molecules are packed more closely than bulk
molecules.
• This causes the liquid to behave as if it had a “skin.”
• Surface tension is the amount of energy required to increase
the surface area of a liquid by a unit amount.
• Stronger intermolecular forces cause higher surface tension.
• Water has a high surface tension (H-bonding)
• Hg(l) has an even higher surface tension (there are very strong
metallic bonds between Hg atoms).
• Cohesive and adhesive forces are at play.
• Cohesive forces are intermolecular forces that bind molecules
to one another.
• Adhesive forces are intermolecular forces that bind molecules
to a surface.
• Illustrate this by looking at the meniscus in a tube filled
with liquid.
• The meniscus is the shape of the liquid surface.
• If adhesive forces are greater than cohesive forces, the liquid
surface is attracted to its container more than the bulk molecules.
Therefore, the meniscus is U-shaped (e.g., water in glass).
• If cohesive forces are greater than adhesive forces, the meniscus
is curved downwards (e.g., Hg(l) in glass)
• Capillary action is the rise of liquids up very narrow tubes.
• The liquid climbs until adhesive and cohesive forces are balanced
by gravity.
FORWARD REFERENCES
• Viscosity of liquid crystals will be discussed in Ch. 12 (section
12.8).
• Viscosity of organic compounds, such as polyhydroxyl alcohols,
will be mentioned in Ch. 25 (section 25.5).
11.4 Phase Changes
• Phase changes are changes of state.
• Matter in one state is converted into another state.
• Sublimation: solid ==> gas.
• Melting or fusion: solid ==> liquid.
• Vaporization: liquid ==> gas.
• Deposition: gas ==> solid.
• Condensation: gas ==> liquid.
• Freezing: liquid ==> solid.
Energy Changes Accompanying Phase Changes
• Energy changes of the system for the above processes are:
melting or fusion: ΔHfus > 0 (endothermic).
• The enthalpy of fusion is known as the heat of fusion.
• vaporization: ΔHvap > 0 (endothermic).
• The enthalpy of vaporization is known as the heat of vaporization.
• sublimation: ΔHsub > 0 (endothermic).
• The enthalpy of sublimation is called the heat of sublimation.
• deposition: ΔHdep < 0 (exothermic).
• condensation: ΔHcon < 0 (exothermic).
• freezing: ΔHfre < 0 (exothermic).
• Generally the heat of fusion (enthalpy of fusion) is less than
heat of vaporization.
• It takes more energy to completely separate molecules than to
partially separate them.
• All phase changes are possible under the right conditions (e.g.,
water sublimes when snow disappears without forming puddles).
• The following sequence is endothermic:
heat solid ==> melt ==> heat liquid ==> boil ==> heat gas
• The following sequence is exothermic:
cool gas ==> condense ==> cool liquid ==> freeze ==> cool
solid
Heating Curves
• Plot of temperature change versus heat added is a heating curve.
• During a phase change adding heat causes no temperature change.
• The added energy is used to break intermolecular bonds rather
than cause a temperature change.
• These points are used to calculate ΔHfus and ΔHvap.
• Supercooling: When a liquid is cooled below its freezing point
and it still remains a liquid.
Critical Temperature and Pressure
• Gases may be liquefied by increasing the pressure at a suitable
temperature.
• Critical temperature is the highest temperature at which a substance
can exist as a liquid.
• Critical pressure is the pressure required for liquefaction
at this critical temperature.
• The greater the intermolecular forces, the easier it is to liquefy
a substance.
• Thus the higher the critical temperature
FORWARD REFERENCES
• Supercritical fluids in green chemistry will be discussed in
Ch. 18 (section 18.7).
• Thermodynamics of phase changes will be further discussed throughout
Ch. 19.
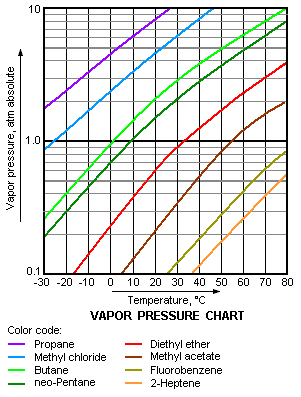
11.5 Vapor Pressure
Explaining Vapor Pressure on the Molecular Level
• Some of the molecules on the surface of a liquid have enough
energy to escape the attraction of the bulk liquid.
• These molecules move into the gas phase.
• As the number of molecules in the gas phase increases, some
of the gas phase molecules strike the surface and return to the liquid.
• After some time the pressure of the gas will be constant.
• A dynamic equilibrium has been established.
• Dynamic equilibrium is a condition in which two opposing processes
occur simultaneously at equal rates.
• In this case, it is the point when as many molecules escape
the surface as strike the surface.
• Vapor pressure of a liquid is the pressure exerted by its vapor
when the liquid and vapor are in dynamic equilibrium.
• The pressure of the vapor at this point is called the equilibrium
vapor pressure.
Volatility, Vapor Pressure, and Temperature ,
• If equilibrium is never established the vapor continues to form.
• Eventually, the liquid evaporates to dryness.
• Liquids that evaporate easily are said to be volatile.
• The higher the temperature, the higher the average kinetic energy,
the faster the liquid evaporates.
Vapor Pressure and Boiling Point
• Liquids boil when the external pressure at the liquid surface
equals the vapor pressure.
• The normal boiling point is the boiling point at 760 mm Hg (1
atm).
• The temperature of the boiling point increases as the external
pressure increases.
• Two ways to get a liquid to boil increase temperature or decrease
pressure.
• Pressure cookers operate at high pressure.
• At high pressure the boiling point of water is higher than at
1 atm.
• Therefore, food is cooked at a higher temperature.
FORWARD REFERENCES
• Vapor pressure reduction of the solvent in a solution—a
colligative property—will be discussed in Ch. 13 (section 13.5).
11.6 Phase Diagrams
• A phase diagram is a plot of pressure vs. temperature summarizing
all equilibria between phases.
• Phase diagrams tell us which phase will exist at a given temperature
and pressure.
• Features of a phase diagram include:
• vapor-pressure curve: generally as temperature increases, vapor
pressure increases.
• critical point: critical temperature and pressure for the gas.
• normal melting point: melting point at 1 atm.
• triple point: temperature and pressure at which all three phases
are in equilibrium.
• Any temperature and pressure combination not on a curve represents
a single phase.
Phase Diagrams of H2O and CO2
• Water:
• In general, an increase in pressure favors the more compact
phase of the material.
• This is usually the solid.
• Water is one of the few substances whose solid form is less
dense than the liquid form.
• The melting point curve for water slopes to the left.
• The triple point occurs at 0.0098 æC and 4.58 mm Hg.
• The normal melting (freezing) point is 0 æC.
• The normal boiling point is 100 æC.
• The critical point is 374 æC and 218 atm.
• Carbon Dioxide:
• The triple point occurs at -56.4 æC and 5.11 atm.
• The normal sublimation point is -78.5 æC. (At 1 atm CO2 sublimes,
it does not melt.)
• The critical point occurs at 31.1 æC and 73 atm.
• Freeze drying: Frozen food is placed in a low pressure (<
4.58 torr) chamber.
• The ice sublimes.
FORWARD REFERENCES
• Phase diagrams for a pure solvent and for a solution of a nonvolatile
solute will be discussed in Ch. 13 (section 13.5).
• Phase equilibria at melting and boiling points will be further
analyzed in Ch. 19.
11.7 Structures of Solids
• A crystalline solid has a well-ordered, definite arrangements
of molecules, atoms, or ions.
• Examples are quartz, diamond, salt, and sugar.
• The intermolecular forces are similar in strength.
• Thus they tend to melt at specific temperatures.
• In an amorphous solid, molecules, atoms or ions do not have
an orderly arrangement.
• Examples are rubber and glass.
• Amorphous solids have intermolecular forces that vary in strength.
• Thus they tend to melt over a range of temperatures.
Unit Cells
• Crystalline solids have an ordered, repeating structure.
• The smallest repeating unit in a crystal is a unit cell.
• The unit cell is the smallest unit with all the symmetry of
the entire crystal.
• The three-dimensional stacking of unit cells is the crystal
lattice.
• Each point in the lattice is a lattice point which represents
an identical environment within the solid.
• There are three types of cubic unit cells.
• Primitive cubic.
• The lattice points are at the corners of a simple cube with
each atom shared by eight unit cells.
• Body-centered cubic (bcc).
• Lattice points occur at the corners of a cube and in addition
there is a lattice point in the center of the body of the cube. The
corner lattice points are shared by eight unit cells, and the center
atom is completely enclosed in one unit cell.
• Face-centered cubic (fcc).
• There are lattice points at the corners of a cube plus one lattice
point in the center of each face of the cube. Eight unit cells share
the corner lattice points and two unit cells share the face lattice
points.
The Crystal Structure of Sodium Chloride
• It has a face-centered cubic lattice.
• There are two equivalent ways of defining this unit cell:
• Cl– (larger) ions at the corners of the cell, or
• Na+ (smaller) ions at the corners of the cell.
• The cation to anion ratio in a unit cell is the same for the
crystal.
• In NaCl each unit cell contains the same number of Na+ and Cl–
ions.
• Note that the unit cell for CaCl2 needs twice as many Cl–
ions as Ca2+ ions.
Close Packing of Spheres
• Crystalline solids have structures that maximize the attractive
forces between particles.
• Their particles can be modeled by spheres.
• Each atom or ion is represented by a sphere.
• Molecular crystals are formed by close packing of the molecules.
• Maximum intermolecular forces in crystals are achieved by the
close packing of spheres.
• A crystal is built up by placing close packed layers of spheres
on top of each other.
• There is only one place for the second layer of spheres.
• There are two choices for the third layer of spheres:
• The third layer eclipses the first (ABAB arrangement).
• This is called hexagonal close packing (hcp).
• The third layer is in a different position relative to the first
(ABCABC arrangement).
• This is called cubic close packing (ccp).
• Note that the unit cell of a ccp crystal is face-centered cubic.
• In both close-packed structures, each sphere is surrounded by
12 other spheres (6 in one plane, 3 above, and 3 below).
• Coordination number is the number of spheres directly surrounding
a central sphere.
• When spheres are packed as closely as possible, there are small
spaces between adjacent spheres (interstitial holes).
• If unequally sized spheres are used, the smaller spheres are
placed in the interstitial holes.
• For example: Li2O
• The larger O2– ions assume the cubic close-packed structure
with the smaller Li+ ions in the holes.
FORWARD REFERENCES
• Coordination numbers will be used in Ch. 23 for transition metal
complexes.
11.8 Bonding in Solids
• The physical properties of crystalline solids depend on the:
• attractive forces between particles and on
• the arrangement of the particles.
Molecular Solids
• Molecular solids consist of atoms or molecules held together
by intermolecular forces.
• Weak intermolecular forces give rise to low melting points.
• Intermolecular forces include dipole–dipole, London dispersion
and H-bonds.
• Molecular solids are usually soft.
• They are often gases or liquids are room temperature.
• Efficient packing of molecules is important (since they are
not regular spheres).
• Molecular solids show poor thermal and electrical conductivity.
• Examples: Ar(s), CH4(s), CO2(s), sucrose.
Covalent-Network Solids
• Covalent-network solids consist of atoms held together, in large
networks or chains, with covalent bonds.
• They have much higher melting points and are much harder than
molecular solids.
• This is a consequence of the strong covalent bonds that connect
the atoms.
• Examples are diamond, graphite, quartz (SiO2), and silicon carbide
(SiC).
• In diamond:
• each C atom has a coordination number of 4.
• each C atom is tetrahedral.
• there is a three-dimensional array of atoms.
• Diamond is hard, and has a high melting point (3550æC).
• In graphite:
• each C atom is arranged in a planar hexagonal ring.
• layers of interconnected rings are placed on top of each other.
• the distance between adjacent C atoms in the same layer is close
to that seen in benzene (1.42 ‰ vs. 1.395 ‰ in benzene).
• electrons move in delocalized orbitals (good conductor).
• the distance between layers is large (3.41 ‰).
• the layers are held together by weak dispersion forces.
• They slide easily past each other.
• Graphite is a good lubricant.
Ionic Solids
• Ionic solids consist of ions held together by ionic bonds.
• They are hard, brittle and have high melting points.
• Ions (spherical) are held together by electrostatic forces of
attraction.
• Recall:
• The larger the charges (q1, q2) and smaller the distance (d)
between ions, the stronger the ionic bond.
• The structure of the ionic solid depends on the charges on the
ions and on the relative sizes of the atoms.
• Examples of some ionic lattice types are:
• NaCl structure.
• Each ion has a coordination number of six.
• It has a face-centered cubic lattice.
• The cation to anion ratio is 1:1.
• Other similar examples: LiF, KCl, AgCl, and CaO.
• CsCl structure.
• Cs+ has a coordination number of eight.
• It is different from the NaCl structure (Cs+ is larger than
Na+).
• The cation to anion ratio is 1:1.
• Zinc blende (ZnS) structure.
• S2– ions adopt a face-centered cubic arrangement.
• Zn2+ ions have a coordination number of four.
• The S2– ions are placed in a tetrahedron around the Zn2+
ions.
• Another example is CuCl.
• Fluorite (CaF2) structure.
• Ca2+ ions are in a face-centered cubic arrangement.
• There are twice as many F– ions as Ca2+ ions in each unit
cell.
• Other examples are BaCl2 and PbF2.
Metallic Solids
• Metallic solids consist entirely of metal atoms.
• Metallic solids are soft or hard.
• They have high melting points.
• They show good electrical and thermal conductivity.
• They are ductile and malleable.
• Examples are all metallic elements (i.e., Al, Cu, Fe, Au)
• Metallic solids have metal atoms in hexagonal close-packed,
face-centered cubic or body-centered cubic arrangements.
• Thus the coordination number for each atom is either 8 or 12.
• A problem that needs to be explained:
• The bonding is too strong to be explained by London dispersion
forces and there are not enough electrons for covalent bonds.
• Resolution:
• The metal nuclei float in a sea of delocalized valence electrons.
• Metals conduct heat and electricity because the valence electrons
are delocalized and are mobile.
X-Ray Diffraction
• When waves are passed through a narrow slit they are diffracted.
• When waves are passed through a diffraction grating (many narrow
slits in parallel) they interact to form a diffraction pattern (areas
of light and dark bands).
• Efficient diffraction occurs when the wavelength of light is
close to the size of the slits.
• The spacing between layers in a crystal is 2–20 ‰,
which is the wavelength range for X-rays.
• X-ray diffraction (X-ray crystallography):
• X-rays are passed through the crystal and are detected on a
photographic plate.
• The photographic plate has one bright spot at the center (incident
beam) as well as a diffraction pattern.
• Each close-packing arrangement produces a different diffraction
pattern.
• Knowing the diffraction pattern, we can calculate the positions
of the atoms required to produce that pattern.
• We calculate the crystal structure based on a knowledge of the
diffraction pattern.
|
Chapter 13
- Properties of Solutions
13.1 The Solution Process
• A solution is a homogeneous mixture of solute and solvent.
• Solutions may be gases, liquids, or solids.
• Each substance present is a component of the solution.
• The solvent is the component present in the largest amount.
• The other components are the solutes.
The Effect of Intermolecular Forces
• Intermolecular forces become rearranged in the process of making
solutions with condensed phases.
• Consider NaCl (solute) dissolving in water (solvent):
• Water molecules orient themselves on the NaCl crystals.
• H-bonds between the water molecules have to be broken.
• NaCl dissociates into Na+ and Cl–.
• Ion–dipole forces form between the Na+ and the negative
end of the water dipole.
• Similar ion–dipole interactions form between the Cl–
and the positive end of the water dipole.
• Such an interaction between solvent and solute is called solvation.
• If water is the solvent, the interaction is called hydration.
Energy Changes and Solution Formation
• There are three steps involving energy in the formation of a
solution:
• Separation of solute molecules (ΔH1),
• Separation of solvent molecules (ΔH2), and
• Formation of solute–solvent interactions (ΔH3).
• We define the enthalpy change in the solution process as:
ΔHsoln = ΔH1 + ΔH2 + ΔH3
• ΔHsoln can either be positive or negative depending on
the intermolecular forces.
• To determine whether ΔHsoln is positive or negative, we
consider the strengths of all solute–solute, solvent–solvent,
and solute–solvent interactions:
• Breaking attractive intermolecular forces is always endothermic.
• ΔH1 and ΔH2 are both positive.
• Forming attractive intermolecular forces is always exothermic.
• ΔH3 is always negative.
• It is possible to have either ΔH3 > (ΔH1 + ΔH2)
or ΔH3 < (ΔH1 + ΔH2).
• Examples:
• MgSO4 added to water has ΔHsoln = –91.2 kJ/mol.
• NH4NO3 added to water has ΔHsoln = + 26.4 kJ/mol.
• MgSO4 is often used in instant heat packs and NH4NO3 is often
used in instant cold packs.
• How can we predict if a solution will form?
• In general, solutions form if the ΔHsoln is negative.
• If ΔHsoln is too endothermic a solution will not form.
• “Rule of thumb”: polar solvents dissolve polar solutes.
• Nonpolar solvents dissolve nonpolar solutes.
• Consider the process of mixing NaCl in gasoline.
• Only weak interactions are possible because gasoline is nonpolar.
• These interactions do not compensate for the separation of ions
from one another.
• Result: NaCl doesn't dissolve to any great extent in gasoline.
• Consider the process of mixing water in octane (C8H18).
• Water has strong H-bonds.
• The energy required to break these H-bonds is not compensated
for by interactions between water and octane.
• Result: water and octane do not mix.
Solution Formation, Spontaneity, and Disorder
• A spontaneous process occurs without outside intervention.
• When the energy of the system decreases (e.g., dropping a book
and allowing it to fall to a lower potential energy), the process is
spontaneous.
• Spontaneous processes tend to be exothermic.
• However, some spontaneous processes do not involve the movement
of the system to a lower energy state (e.g., an endothermic reaction).
• Some endothermic processes occur spontaneously.
• The amount of randomness or disorder of the system is given
by its entropy.
• In most cases, solution formation is favored by the increase
in entropy that accompanies mixing.
• Example: A mixture of CCl4 and C6H14 is less ordered than the
two separate liquids.
• Therefore, they spontaneously mix even though ΔHsoln is
very close to zero.
• A solution will form unless the solute–solute or solvent–solvent
interactions are too strong relative to solute–solvent interactions.
Solution Formation and Chemical Reactions
• Some solutions form by physical processes and some by chemical
processes.
• Consider:
Ni(s) + 2HCl(aq) ‡ NiCl2(aq) + H2(g)
• Note that the chemical form of the substance being dissolved
has changed during this process (Ni ‡ NiCl2)
• When all the water is removed from the solution, no Ni is found,
only NiCl2•6H2O remains.
• Therefore, the dissolution of Ni in HCl is a chemical process.
• By contrast:
NaCl(s) + H2O (l) ‡ Na+(aq) + Cl–(aq).
• When the water is removed from the solution, NaCl is found.
• Therefore, NaCl dissolution is a physical process.
FORWARD REFERENCES
• Hydrolysis of metal ions will be brought up again in Ch. 16
(section 16.11).
• Thermodynamics of processes will be further discussed throughout
Ch. 19.
• Rust is a hydrate (Ch. 20, section 20.8).
• Solution alloys will be further described in Ch. 23 (section
23.6).
13.2 Saturated Solutions and Solubility
• As a solid dissolves, a solution forms:
• Solute + solvent ==> solution
• The opposite process is crystallization.
• Solution ==> solute + solvent
• If crystallization and dissolution are in equilibrium with undissolved
solute, the solution is saturated.
• There will be no further increase in the amount of dissolved
solute.
• Solubility is the amount of solute required to form a saturated
solution.
• A solution with a concentration of dissolved solute that is
less than the solubility is said to be unsaturated.
• A solution is said to be supersaturated if more solute is dissolved
than in a saturated solution.
FORWARD REFERENCES
• Solubility equilibria will be further discussed in Ch. 17 (section
17.4).
• Various crystalline substances (anhydrous and hydrated) for
transition metal compounds will be described throughout Ch. 23.
13.3 Factors Affecting Solubility
• The tendency of a substance to dissolve in another depends on:
• the nature of the solute.
• the nature of the solvent.
• the temperature.
• the pressure (for gases).
Solute–Solvent Interactions
• Intermolecular forces are an important factor in determining
solubility of a solute in a solvent.
• The stronger the attraction between solute and solvent molecules,
the greater the solubility.
• For example, polar liquids tend to dissolve in polar solvents.
• Favorable dipole–dipole interactions exist (solute–solute,
solvent–solvent, and solute–solvent).
• Pairs of liquids that mix in any proportions are said to be
miscible.
• Example: Ethanol and water are miscible liquids.
• In contrast, immiscible liquids do not mix significantly.
• Example: Gasoline and water are immiscible.
• Consider the solubility of alcohols in water.
• Water and ethanol are miscible because the broken hydrogen bonds
in both pure liquids are re-established in the mixture.
• However, not all alcohols are miscible with water.
• Why?
• The number of carbon atoms in a chain affects solubility.
• The greater the number of carbons in the chain, the more the
molecule behaves like a hydrocarbon.
• Thus, the more C atoms in the alcohol, the lower its solubility
in water.
• Increasing the number of –OH groups within a molecule
increases its solubility in water.
• The greater the number of –OH groups along the chain,
the more solute-water H-bonding is possible.
• Generalization: “like dissolves like”.
• Substances with similar intermolecular attractive forces tend
to be soluble in one another.
• The more polar bonds in the molecule, the better it dissolves
in a polar solvent.
• The less polar the molecule the less likely it is to dissolve
in a polar solvent and the more likely it is to dissolve in a nonpolar
solvent.
• Network solids do not dissolve because the strong intermolecular
forces in the solid are not reestablished in any solution.
Pressure Effects
• The solubility of a gas in a liquid is a function of the pressure
of the gas over the solution.
• Solubilities of solids and liquids are not greatly affected
by pressure.
• With higher gas pressure, more molecules of gas are close to
the surface of the solution and the probability of a gas molecule striking
the surface and entering the solution is increased.
• Therefore, the higher the pressure, the greater the solubility.
• The lower the pressure, the smaller the number molecules of
gas close to the surface of the solution resulting in a lower solubility.
• The solubility of a gas is directly proportional to the partial
pressure of the gas above the solution.
• This statement is called Henry's law.
• Henry's law may be expressed mathematically as: Sg=kPg
• Where Sg is the solubility of gas, Pg the partial pressure,
k = Henry’s law constant.
• Note that the Henry's law constant differs for each solute–solvent
pair and differs with temperature.
• An application of Henry's law is the preparation of carbonated
soda.
• Carbonated beverages are bottled under PCO2 > 1 atm.
• As the bottle is opened, PCO2 decreases and the solubility of
CO2 decreases.
• Therefore, bubbles of CO2 escape from solution.
Temperature Effects
• Experience tells us that sugar dissolves better in warm water
than in cold water.
• As temperature increases, solubility of solids generally increases.
• Sometimes solubility decreases as temperature increases [e.g.,
Ce2(SO4)3].
• Experience tells us that carbonated beverages go flat as they
get warm.
• Gases are less soluble at higher temperatures.
• An environmental application of this is thermal pollution.
• Thermal pollution: if lakes get too warm, CO2 and O2 become
less soluble and are not available for plants or animals.
• Fish suffocate.
FORWARD REFERENCES
• The dynamic equilibrium between a solid solute and its solution
will be mentioned in Ch. 14 (section 14.7).
• Factors affecting solubility will be discussed in detail in
Ch. 17 (section 17.5).
• Temperature effects on solubility of NaCl will be mentioned
in Ch. 19 (section 19.7).
• Reactions involving CO2, HCO3- and H2CO3 will be discussed in
Ch. 22 (section 22.9).
• Solubility of organic substances in polar solvents will be mentioned
in Ch. 25 (section 25.1).
13.4 Ways of Expressing Concentration
• All methods involve quantifying the amount of solute per amount
of solvent (or solution).
• Concentration may be expressed qualitatively or quantitatively.
• The terms dilute and concentrated are qualitative ways to describe
concentration.
• A dilute solution has a relatively small concentration of solute.
• A concentrated solution has a relatively high concentration
of solute.
• Quantitative expressions of concentration require specific information
regarding such quantities as masses, moles, or liters of the solute,
solvent, or solution.
• The most commonly used expressions for concentration are:
• mass percentage.
• mole fraction.
• molarity.
• molality.
Mass Percentage, ppm, and ppb
• Mass percentage is one of the simplest ways to express concentration.
• By definition: (wouldn't copy -- in book)
• Similarly, parts per million (ppm) can be expressed as the number
of mg of solute per kilogram of solution.
• By definition: (wouldn't copy -- in book)
• The density of a very dilute aqueous solution is similar to
that of pure water (1g/ml)
• If the density of the solution is 1g/ml, then 1 ppm = 1 mg solute
per liter of solution.
• We can extend this again!
• Parts per billion (ppb) can be expressed as the number of µg
of solute per kilogram of solution.
• By definition: (wouldn't copy -- in book)
• If the density of the solution is 1g/ml, then 1 ppb = 1 µg
solute per liter of solution.
Mole Fraction, Molarity, and Molality
• Common expressions of concentration are based on the number
of moles of one or more components.
• Recall that mass can be converted to moles using the molar mass.
• Recall:
• Note that mole fraction has no units.
• Note that mole fractions range from 0 to 1.
• Recall:
• Note that molarity will change with a change in temperature
(as the solution volume increases or decreases).
• We can define molality (m), yet another concentration unit:
• Molality does not vary with temperature.
• Note that converting between molarity (M) and molality (m) requires
density.
• The molarity and molality of dilute solutions are often very
similar.
FORWARD REFERENCES
• Molar concentrations will be used in rate law expressions in
Ch. 14.
• Molar concentrations will be used in equilibrium constant and
reaction quotient expressions in Ch. 15, 16, 17, 19, and 20.
• Concentrations (ppm) of trace constituents in mixtures will
be mentioned in Ch. 18 (section 18.1).
13.5 Colligative Properties
• Colligative properties depend on number of solute particles.
• There are four colligative properties to consider:
• vapor pressure lowering (Raoult's Law).
• boiling point elevation.
• freezing point depression.
• osmotic pressure.
Lowering the Vapor Pressure
• Consider a volatile liquid in a closed container.
• After a period of time an equilibrium will be established between
the liquid and its vapor.
• The partial pressure exerted by the vapor is the vapor pressure.
• Nonvolatile solutes (with no measurable vapor pressure) reduce
the ability of the surface solvent molecules to escape the liquid.
• Therefore, vapor pressure is lowered.
• The amount of vapor pressure lowering depends on the amount
of solute.
• Raoult’s law quantifies the extent to which a nonvolatile
solute lowers the vapor pressure of the solvent.
• If PA is the vapor pressure with solute, PæA is the vapor pressure
of the pure solvent, and CA is the mole fraction of A, then
• An ideal solution is one that obeys Raoult’s law.
• Real solutions show approximately ideal behavior when:
• the solute concentration is low.
• the solute and solvent have similarly sized molecules.
• the solute and solvent have similar types of intermolecular
attractions.
• Raoult’s law breaks down when the solvent–solvent
and solute–solute intermolecular forces are much greater or weaker
than solute–solvent intermolecular forces.
Boiling-Point Elevation
• A nonvolatile solute lowers the vapor pressure of a solution.
• At the normal boiling point of the pure liquid, the solution
has a has a vapor pressure less than 1 atm.
• Therefore, a higher temperature is required to reach a vapor
pressure of 1 atm for the solution (ΔTb).
• The molal boiling-point-elevation constant, Kb, expresses how
much ΔTb changes with molality, m: ΔTb = Kbm
• The nature of the solute (electrolyte vs. nonelectrolyte) will
impact the colligative molality of the solute.
Freezing-Point Depression
• When a solution freezes, crystals of almost pure solvent are
formed first.
• Solute molecules are usually not soluble in the solid phase
of the solvent.
• Therefore, the triple point occurs at a lower temperature because
of the lower vapor pressure for the solution.
• The melting-point (freezing-point) curve is a vertical line
from the triple point.
• Therefore, the solution freezes at a lower temperature (ΔTf)
than the pure solvent.
• The decrease in freezing point (ΔTf) is directly proportional
to molality.
• Kf is the molal freezing-point-depression constant.
ΔTf = Kfm
• Values of Kf and Kb for most common solvents can be found in
Table 13.4.
Osmosis
• Semipermeable membranes permit passage of some components of
a solution.
• Often they permit passage of water but not larger molecules
or ions.
• Examples of semipermeable membranes are cell membranes and cellophane.
• Osmosis is the net movement of a solvent from an area of low
solute concentration to an area of high solute concentration.
• Consider a U-shaped tube with a two liquids separated by a semipermeable
membrane.
• One arm of the tube contains pure solvent.
• The other arm contains a solution.
• There is movement of solvent in both directions across a semipermeable
membrane.
• As solvent moves across the membrane, the fluid levels in the
arms become uneven.
• The vapor pressure of solvent is higher in the arm with pure
solvent.
• Eventually the pressure difference due to the difference in
height of liquid in the arms stops osmosis.
• Osmotic pressure, p, is the pressure required to prevent osmosis.
• Osmotic pressure obeys a law similar in form to the ideal-gas
law.
• For n moles, V= volume, M= molarity, R= the ideal gas constant,
and an absolute temperature, T, the osmotic pressure is:
pV = nRT
• Two solutions are said to be isotonic if they have the same
osmotic pressure.
• Hypotonic solutions have a lower p, relative to a more concentrated
solution.
• Hypertonic solutions have a higher p, relative to a more dilute
solution.
• We can illustrate this with a biological system: red blood cells.
• Red blood cells are surrounded by semipermeable membranes.
• If red blood cells are placed in a hypertonic solution (relative
to intracellular solution), there is a lower solute concentration in
the cell than the surrounding tissue.
• Osmosis occurs and water passes through the membrane out of
the cell.
• The cell shrivels up.
• This process is called crenation.
• If red blood cells are placed in a hypotonic solution, there
is a higher solute concentration in the cell than outside the cell.
• Osmosis occurs and water moves into the cell.
• The cell bursts (hemolysis).
• To prevent crenation or hemolysis, IV (intravenous) solutions
must be isotonic relative to the intracellular fluids of cells.
• Everyday examples of osmosis include:
• If a cucumber is placed in NaCl solution, it will lose water
to shrivel up and become a pickle.
• A limp carrot placed in water becomes firm because water enters
via osmosis.
• Eating large quantities of salty food causes retention of water
and swelling of tissues (edema).
• Water moves into plants, to a great extent, through osmosis.
• Salt may be added to meat (or sugar added to fruit) as a preservative.
• Salt prevents bacterial infection: A bacterium placed on the
salt will lose water through osmosis and die.
• Active transport is the movement of nutrients and waste material
through a biological membrane against a concentration gradient.
• Movement is from an area of low concentration to an area of
high concentration.
• Active transport is not spontaneous.
• Energy must be expended by the cell to accomplish this.
Determination of Molar Mass
• Any of the four colligative properties may be used to determine
molar mass.
FORWARD REFERENCES
• Desalination via reverse osmosis will be described in Ch. 18
(section 18.5).
13.6 Colloids
• Colloids or colloidal dispersions are suspensions in which the
suspended particles are larger than molecules but too small to separate
out of the suspension due to gravity.
• Particle size: 5 to 1000 nm.
• There are several types of colloids:
• aerosol: gas + liquid or solid (e.g., fog and smoke),
• foam: liquid + gas (e.g., whipped cream),
• emulsion: liquid + liquid (e.g., milk),
• sol: liquid + solid (e.g., paint),
• solid foam: solid + gas (e.g., marshmallow),
• solid emulsion: solid + liquid (e.g., butter),
• solid sol: solid + solid (e.g., ruby glass).
• The Tyndall effect is the ability of colloidal particles to
scatter light.
• The path of a beam of light projected through a colloidal suspension
can be seen through the suspension.
Hydrophilic and Hydrophobic Colloids
• Focus on colloids in water.
• Water-loving colloids are hydrophilic.
• Water-hating colloids are hydrophobic.
• In the human body, large biological molecules such as proteins
are kept in suspension by association with surrounding water molecules.
• These macromolecules fold up so that hydrophobic groups are
away from the water (inside the folded molecule).
• Hydrophilic groups are on the surface of these molecules and
interact with solvent (water) molecules.
• Typical hydrophilic groups are polar (containing C–O,
O–H, N–H bonds) or charged.
• Hydrophobic colloids need to be stabilized in water.
• One way to stabilize hydrophobic colloids is to adsorb ions
on their surface.
• Adsorption: when something sticks to a surface we say that it
is adsorbed.
• If ions are adsorbed onto the surface of a colloid, the colloid
appears hydrophilic and is stabilized in water.
• Consider a small drop of oil in water.
• Add a small amount of sodium stearate.
• Sodium stearate has a long hydrophobic hydrocarbon tail and
a small hydrophilic head.
• The hydrophobic tail can be absorbed into the oil drop, leaving
the hydrophilic head on the surface.
• The hydrophilic heads then interact with the water and the oil
drop is stabilized in water.
• A soap acts in a similar fashion.
• Soaps are molecules with long hydrophobic tails and hydrophilic
heads that remove dirt by stabilizing the colloid in water.
• Most dirt stains on people and clothing are oil based.
• Biological application of this principle:
• The gallbladder excretes a fluid called bile.
• Bile contains substances (bile salts) that form an emulsion
with fats in our small intestine.
• Emulsifying agents help form an emulsion.
• Emulsification of dietary fats and fat-soluble vitamins is important
in their absorption and digestion by the body.
Removal of Colloid Particles
• We often need to separate colloidal particles from the dispersing
medium.
• This may be problematic.
• Colloid particles are too small to be separated by physical
means (e.g., filtration).
• However, colloid particles often may be coagulated (enlarged)
until they can be removed by filtration.
• There are various methods of coagulation.
• Colloid particles move more rapidly when the colloidal dispersion
is heated, increasing the number of collisions. The particles stick
to each other when they collide.
• Adding an electrolyte neutralizes the surface charges on the
colloid particles.
• A biological application of another approach to separating colloidal
particles from the suspending medium is dialysis.
• In dialysis a semipermeable membrane is used to separate ions
from colloidal particles.
• In kidney dialysis, the blood is allowed to pass through a semipermeable
membrane immersed in a washing solution.
• The washing solution is isotonic in ions that must be retained.
• The washing solution does not have the waste products that are
found in the blood.
• Wastes therefore dialyze out of the blood (move from the blood
into the washing solution).
• The "good" ions remain in the blood.
FORWARD REFERENCES
• Adsorption and absorption in heterogeneous catalysis will be
mentioned in Ch. 14 (section 14.7).
• A model of hemoglobin in blood will be provided in chapters
24 and 25 (sections 24.2 and 25.9, respectively).
• Surfactant organic molecules will be mentioned again in Ch.
25 (section 25.1). |